Peptide-induced super-assembly of biocatalytic metal–organic frameworks for programmed enzyme cascades†
Abstract
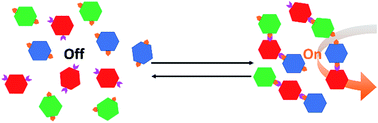
Despite the promise of metal–organic frameworks (MOFs) as functional matrices for enzyme stabilization, the development of a stimulus-responsive approach to induce a multi-enzyme cascade reaction in MOFs remains a critical challenge. Here, a novel method using peptide-induced super-assembly of MOFs is developed for programmed enzyme cascade reactions on demand. The super-assembled MOF particles containing different enzymes show remarkable 7.3-fold and 4.4-fold catalytic activity enhancements for the two-enzyme and three-enzyme cascade reactions, respectively, as compared with the unassembled MOF nanoparticles. Further digestion of the coiled-coil forming peptides on the MOF surfaces leads to the MOF superstructure disassembly and the programmed enzyme cascade reaction being “switched-off”. Research on these stimuli-responsive materials with controllable and predictable biocatalytic functions/properties provide a concept to facilitate the fabrication of next-generation smart materials based on precision chemistry.


 Please wait while we load your content...
Please wait while we load your content...