Selective single C–F bond arylation of trifluoromethylalkene derivatives†
Abstract
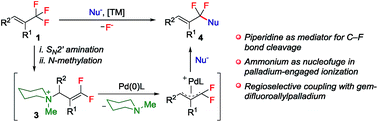
A strategically novel single C–F bond functionalization of CF3-derived molecules, which shows a prominent advantage for the expedient construction of difluoromethylene-bridged organic scaffolds, is disclosed. The reported protocol consists of SN2′ amination, N-alkylation and palladium-catalyzed allylic substitution reactions, which enables straightforward arylation and alkenylation of vinyltrifluoromethane derivatives. Furthermore, this strategy is characterized by its broad substrate scope with respect to both CF3-alkene and arylboronic acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...