A reactive coordinatively saturated Mo(iii) complex: exploiting the hemi-lability of tris(tert-butoxy)silanolate ligands†
Abstract
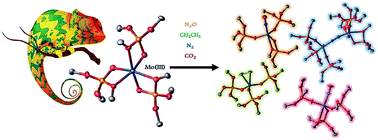
Coordinatively unsaturated Mo(III) complexes have been identified as highly reactive species able to activate dinitrogen without the need for a sacrificial reducing agent. Here, we report a coordinatively saturated octahedral Mo(III) complex stabilized by κ2-tris(tert-butoxy)silanolate ligands, which is yet highly reactive towards dinitrogen and small molecules. The combined high stability and activity are ascribed to the dual binding mode of the tris(tert-butoxy)silanolate ligands that allow unlocking a coordination site in the presence of reactive small molecules to promote their activation at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...