Acylation-coupled lipophilic induction of polarisation (Acyl-cLIP): a universal assay for lipid transferase and hydrolase enzymes†
Abstract
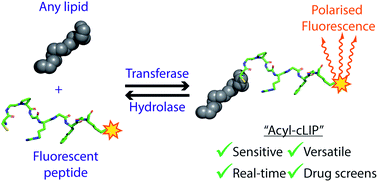
Posttranslational attachment of lipids to proteins is important for many cellular functions, and the enzymes responsible for these modifications are implicated in many diseases, from cancer to neurodegeneration. Lipid transferases and hydrolases are increasingly tractable therapeutic targets, but present unique challenges for high-throughput biochemical enzyme assays which hinder development of new inhibitors. We present Acylation-coupled Lipophilic Induction of Polarisation (Acyl-cLIP) as the first universally applicable biochemical lipidation assay, exploiting the hydrophobic nature of lipidated peptides to drive a polarised fluorescence readout. Acyl-cLIP allows sensitive, accurate, real-time measurement of S- or N-palmitoylation, N-myristoylation, S-farnesylation or S-geranylgeranylation. Furthermore, it is applicable to transfer and hydrolysis reactions, and we demonstrate its extension to a high-throughput screening format. We anticipate that Acyl-cLIP will greatly expedite future drug discovery efforts against these challenging targets.


 Please wait while we load your content...
Please wait while we load your content...