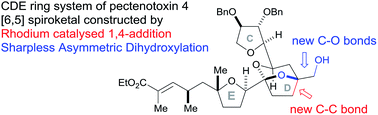
Rhodium-catalysed vinyl 1,4-conjugate addition coupled with Sharpless asymmetric dihydroxylation in the synthesis of the CDE ring fragment of pectenotoxin-4†
Abstract
Our synthesis of the CDE ring fragment of pectenotoxin-4 utilised two key steps to make the complex bicyclic ketal unit: (i) a rhodium-catalysed vinyl group 1,4-addition as the major C–C bond forming step; (ii) a stereoselective Sharpless Asymmetric Dihydroxylation (SAD) of the resulting 1,1-disubstituted homoallylic alcohol. Subsequent acid-catalysed cyclisation afforded the desired [5,6]-bicyclic ketal of the target molecule. This methodology was shown to be compatible with the desired E ring fragment 35 in order to construct the CDE fragment 37 of pectenotoxin-4.


 Please wait while we load your content...
Please wait while we load your content...