Mesoporous gold nanospheres via thiolate–Au(i) intermediates†
Abstract
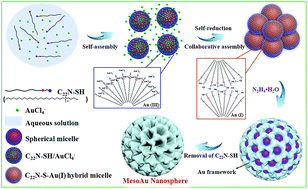
Mesoporous gold (mesoAu) nanospheres support enhanced (electro)catalytic performance owing to their three-dimensional (3D) interior mesochannels that expose abundant active sites and facilitate electron/mass transfers. Although various porous Nanostructured Au has been fabricated by electrochemical reduction, alloying–dealloying and hard/soft templating methods, successful synthesis of mesoAu nanospheres with tailorable sizes and porosities remains a big challenge. Here we describe a novel surfactant-directed synthetic route to fabricate mesoAu nanospheres with 3D interconnected mesochannels by using the amphiphilic surfactant of C22H45N+(CH3)2–C3H6–SH (Cl−) (C22N–SH) as the mesopore directing agent. C22N–SH can not only self-reduce trivalent Au(III)Cl4− to monovalent Au(I), but also form polymeric C22N–S–Au(I) intermediates via covalent bonds. These C22N–S–Au(I) intermediates facilitate the self-assembly into spherical micelles and inhibit the mobility of Au precursors, enabling the crystallization nucleation and growth of the mesoAu nanospheres via in situ chemical reduction. The synthetic strategy can be further extended to tailor the sizes/porosities and surface optical properties of the mesoAu nanospheres. The mesoAu nanospheres exhibit remarkably enhanced mass/specific activity and improved stability in methanol electrooxidation, demonstrating far better performance than non-porous Au nanoparticles and previously reported Au nanocatalysts. The synthetic route differs markedly from other long-established soft-templating approaches, providing a new avenue to grow metal nanocrystals with desirable nanostructures and functions.


 Please wait while we load your content...
Please wait while we load your content...