Palladium-catalyzed enantioselective decarboxylative allylic alkylation of fully substituted N-acyl indole-derived enol carbonates†
Abstract
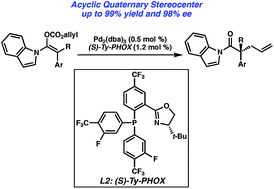
The first enantioselective palladium-catalyzed decarboxylative allylic alkylation of fully substituted N-acyl indole-derived enol carbonates forming acyclic all-carbon quaternary stereocenters is reported. Excellent yields up to 99% and enantioselectivities up to 98% ee are obtained through the use of a new electron-deficient phosphinoxazoline (PHOX) ligand. Control of substrate enolate geometry is crucial for high selectivity. The obtained α-quaternary N-acyl indoles are formal ester equivalents, and represent a useful handle for further synthetic transformations.


 Please wait while we load your content...
Please wait while we load your content...