Isolated α-turn and incipient γ-helix†
Abstract
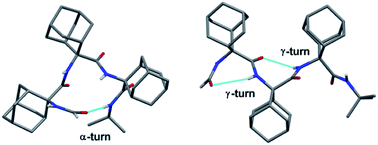
The unique abilities of homo-oligo-adamantyl peptides to adopt α- and γ-turn conformations are demonstrated by X-ray diffraction, and NMR and FT-IR absorption spectroscopies. Assembled by an Ugi multiple component reaction strategy, Nα-formyl-adamantyl tripeptide iso-propyl and tert-butyl amides are respectively found to adopt an isolated α-turn and an incipient γ-helix conformation by X-ray diffraction crystallography. The shortest example of a single α-turn with ideal geometry is observed in the crystalline state. In solution both peptides predominantly assume γ-helical structures.


 Please wait while we load your content...
Please wait while we load your content...