A K+-promoted Diels–Alder reaction by using a self-assembled macrocyclic boronic ester containing two crown ether moieties†
Abstract
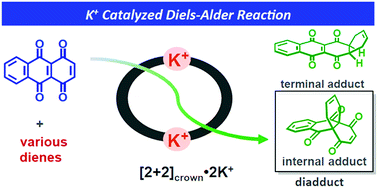
A K+-promoted Diels–Alder reaction of 1,4,9,10-anthradiquinone with various dienes is achieved in the presence of a self-assembled macrocyclic boronic ester [2+2]crown containing two crown ether moieties. The reaction rate is remarkably accelerated (up to 206-fold) compared to that in the absence of the promoter. Furthermore, the reaction proceeds regioselectively to yield an internal adduct. The self-assembly protocol was also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...