Boosting the circularly polarized luminescence of small organic molecules via multi-dimensional morphology control†
Abstract
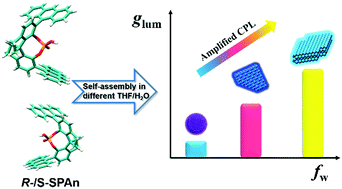
Achieving a higher dissymmetry factor is a crucial issue in developing circularly polarized luminescence (CPL) materials. Here, by tailoring the solvent composition and the morphology of the same chiral emissive small molecules (R- or S-SPAn), circularly polarized emission with a boosted dissymmetry factor (two orders) was realized. It was found that by regulating the water fraction in the mixed THF/H2O, we were able to achieve kinetic control over association of chiral emissive R- or S-SPAn into various nanostructures with 0D nanospheres, 2D nanoflakes and 3D stacked nanoflakes. These nanostructures are all CPL active. Remarkably, the dissymmetry factors of the nanostructures were significantly enhanced compared to those of the molecules and further boosted in different morphologies, from ∼10−4 (0D nanospheres) to 10−3 (2D flake) to ∼10−2 (3D nanoflakes). The enlarged glum value could be assigned to a good packing induced strong luminescence of an excimer. This strategy provides an efficient way to fabricate higher dissymmetry factor CPL organic nanomaterials by only changing the supramolecular architectures while using the same chiral small molecules.
- This article is part of the themed collections: In celebration of Chinese New Year and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...