Ionization of gold (γ-methoxy)vinyl complexes generates reactive gold vinyl carbene complexes†
Abstract
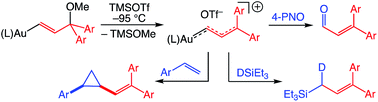
Cationic gold vinyl carbene/allylic cation complexes of the form (E)-[(L)AuC(H)C(H)CAr2]+ OTf− {L = IPr, Ar = Ph [(E)-5a], L = IPr, Ar = 4-C6H4OMe [(E)-5b], L = P(t-Bu)2o-biphenyl, Ar = 4-C6H4OMe [(E)-5c]} were generated in solution via Lewis acid-mediated ionization of the corresponding gold (γ-methoxy)vinyl complexes (E)-(L)AuC(H)C(H)C(OMe)Ar2 at or below −95 °C. Complexes (E)-5b and (E)-5c were fully characterized in solution employing multinuclear NMR spectroscopy, which established the predominant contribution of the aurated allylic cation resonance structure and the significant distribution of positive charge into the γ-anisyl rings. Complex (E)-5b reacted rapidly at −95 °C with neutral two-electron, hydride, and oxygen atom donors exclusively at the C1 position of the vinyl carbene moiety and with p-methoxystyrene to form the corresponding vinylcyclopropane. In the absence of nucleophile (E)-5a decomposed predominantly via intermolecular carbene dimerization whereas formation of 1-aryl-5-methoxy indene upon ionization of (Z)-(IPr)AuC(H)C(H)C(OMe)(4-C6H4OMe)2 [(Z)-6b] implicated an intramolecular Friedel–Crafts or electrocyclic Nazarov pathway for the decomposition of the unobserved vinyl carbene complex (Z)-[(IPr)AuC(H)C(H)C(4-C6H4OMe)2]+ OTf− [(Z)-5b].


 Please wait while we load your content...
Please wait while we load your content...