Synthesis of aminyl biradicals by base-induced Csp3–Csp3 coupling of cationic azo dyes†
Abstract
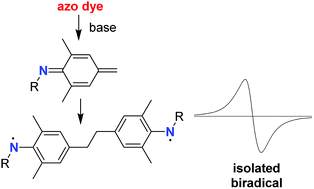
The synthesis of the industrially important polymer parylene is achieved by polymerization of p-quinodimethane (p-QDM). The polymerization is thought to proceed via a biradical p-QDM dimer, but isolation or characterization of such a biradical has remained elusive. Here, we describe the synthesis of an aza-analogue of this p-QDM dimer. The biradical is formed by base-induced dimerization of an azoimidazolium dye. Due to the presence of sterically shielded aminyl radicals instead of terminal H2Ċ groups, the stability of this dimer is sufficient for analyses by ESR spectroscopy and X-ray crystallography. A similar Csp3–Csp3 coupling was observed for an azotriazolium dye, suggesting that base-induced C–C coupling reactions can be realized for different types of azo dyes.


 Please wait while we load your content...
Please wait while we load your content...