Stereoselective total synthesis of parthenolides indicates target selectivity for tubulin carboxypeptidase activity†
Abstract
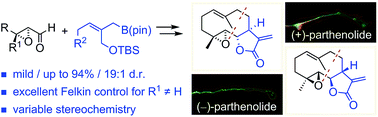
The 2-(silyloxymethyl)allylboration of aldehydes was established to enable stereoselective access to α-(exo)-methylene γ-butyrolactones under mild conditions. Acid-labile functionality and chiral carbonyl compounds are tolerated. Excellent asymmetric induction was observed for β,β′-disubstituted α,β-epoxy aldehydes. These findings led to the enantioselective total synthesis of the sesquiterpene natural product (−)-parthenolide, its unnatural (+)-enantiomer, and diastereoisomers. Among all the isomers tested in cell culture, only (−)-parthenolide showed potent inhibition of microtubule detyrosination in living cells, confirming its exquisite selectivity on tubulin carboxypeptidase activity. On the other hand, the anti-inflammatory activity of the parthenolides was weaker and less selective with regard to compound stereochemistry.


 Please wait while we load your content...
Please wait while we load your content...