Nickel-catalyzed C–H alkylation of indoles with unactivated alkyl chlorides: evidence of a Ni(i)/Ni(iii) pathway†
Abstract
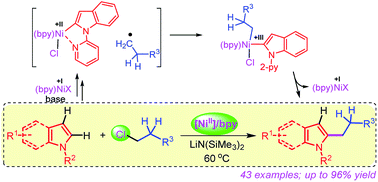
A mild and efficient nickel-catalyzed method for the coupling of unactivated primary and secondary alkyl chlorides with the C–H bond of indoles and pyrroles is described which demonstrates a high level of chemo and regioselectivity. The reaction tolerates numerous functionalities, such as halide, alkenyl, alkynyl, ether, thioether, furanyl, pyrrolyl, indolyl and carbazolyl groups including acyclic and cyclic alkyls under the reaction conditions. Mechanistic investigation highlights that the alkylation proceeds through a single-electron transfer (SET) process with Ni(I)-species being the active catalyst. Overall, the alkylation follows a Ni(I)/Ni(III) pathway involving the rate-influencing two-step single-electron oxidative addition of alkyl chlorides.


 Please wait while we load your content...
Please wait while we load your content...