Photocatalytic carbanion generation – benzylation of aliphatic aldehydes to secondary alcohols†
Abstract
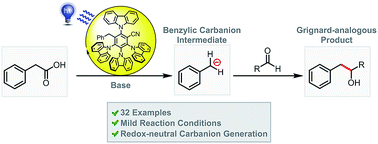
We present a redox-neutral method for the photocatalytic generation of carbanions. Benzylic carboxylates are photooxidized by single electron transfer; immediate CO2 extrusion and reduction of the in situ formed radical yields a carbanion capable of reacting with aliphatic aldehydes as electrophiles giving the Grignard analogous reaction product.


 Please wait while we load your content...
Please wait while we load your content...