Selective coordination of three transition metal ions within a coiled-coil peptide scaffold†
Abstract
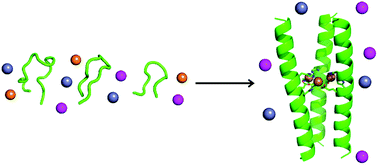
Designing peptides that fold and assemble in response to metal ions tests our understanding of how peptide folding and metal binding influence one another. Here, histidine residues are introduced into the hydrophobic core of a coiled-coil trimer, generating a peptide that self-assembles upon the addition of metal ions. HisAD, the resulting peptide, is unstructured in the absence of metal and folds selectively to form an α-helical construct upon complexation with Cu(II) and Ni(II) but not Co(II) or Zn(II). The structure, and metal-binding ability, of HisAD is probed using a combination of circular dichroism (CD) spectroscopy, analytical ultracentrifugation (AUC), nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography. These show the peptide is trimeric and binds to both Cu(II) and Ni(II) in a 1 : 1 ratio with the histidine residues involved in the metal coordination, as designed. The X-ray crystal structure of the HisAD-Cu(II) complex reveals the trimeric HisAD peptide coordinates three Cu(II) ions; this is the first example of such a structure. Additionally, HisAD demonstrates an unprecedented discrimination between transition metal ions, the basis of which is likely to be related to the stability of the peptide-metal complexes formed.


 Please wait while we load your content...
Please wait while we load your content...