Catalytic reduction of aryl trialkylammonium salts to aryl silanes and arenes†
Abstract
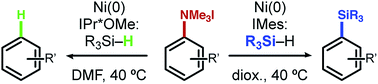
A new approach for the reduction of aryl ammonium salts to arenes or aryl silanes using nickel catalysis is reported. This method displays excellent ligand-controlled selectivity based on the N-heterocyclic carbene (NHC) ligand employed. Utilizing a large NHC in non-polar solvents generates aryl silanes, while small NHCs in polar solvents promote reduction to arenes. Several classes of aryl silanes can be accessed from simple aniline building blocks, including those useful for cross-couplings, oxidations, and halogenations. The reaction conditions are mild, functional group tolerant, and provide efficient access to a variety of benzene derivatives.


 Please wait while we load your content...
Please wait while we load your content...