IRMOF-74(n)–Mg: a novel catalyst series for hydrogen activation and hydrogenolysis of C–O bonds†
Abstract
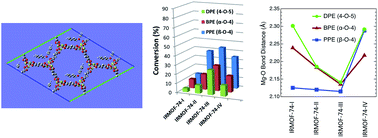
Metal–Organic Frameworks (MOFs) that catalyze hydrogenolysis reactions are rare and there is little understanding of how the MOF, hydrogen, and substrate molecules interact. In this regard, the isoreticular IRMOF-74 series, two of which are known catalysts for hydrogenolysis of aromatic C–O bonds, provides an unusual opportunity for systematic probing of these reactions. The diameter of the 1D open channels can be varied within a common topology owing to the common secondary building unit (SBU) and controllable length of the hydroxy-carboxylate struts. We show that the first four members of the IRMOF-74(Mg) series are inherently catalytic for aromatic C–O bond hydrogenolysis and that the conversion varies non-monotonically with pore size. These catalysts are recyclable and reusable, retaining their crystallinity and framework structure after the hydrogenolysis reaction. The hydrogenolysis conversion of phenylethylphenyl ether (PPE), benzylphenyl ether (BPE), and diphenyl ether (DPE) varies as PPE > BPE > DPE, consistent with the strength of the C–O bond. Counterintuitively, however, the conversion also follows the trend IRMOF-74(III) > IRMOF-74(IV) > IRMOF-74(II) > IRMOF-74(I), with little variation in the corresponding selectivity. DFT calculations suggest the unexpected behavior is due to much stronger ether and phenol binding to the Mg(II) open metal sites (OMS) of IRMOF-74(III), resulting from a structural distortion that moves the Mg2+ ions toward the interior of the pore. Solid-state 25Mg NMR data indicate that both H2 and ether molecules interact with the Mg(II) OMS and hydrogen–deuterium exchange reactions show that these MOFs activate dihydrogen bonds. The results suggest that both confinement and the presence of reactive metals are essential for achieving the high catalytic activity, but that subtle variations in pore structure can significantly affect the catalysis. Moreover, they challenge the notion that simply increasing MOF pore size within a constant topology will lead to higher conversions.


 Please wait while we load your content...
Please wait while we load your content...