Conversion of two stereocenters to one or two chiral axes: atroposelective synthesis of 2,3-diarylbenzoindoles†
Abstract
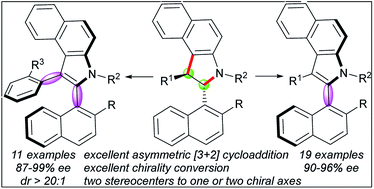
Central-to-axial chirality conversion provides efficient access to axially chiral compounds, and several examples regarding the conversion of one, two or four stereocenters to one axis have been reported. Herein, we report the conversion of two stereocenters to one or two chiral axes for the first time. In this study, a new class of enantiomerically enriched 2,3-diarylbenzoindoles was efficiently synthesized using a chiral phosphoric acid-catalyzed [3 + 2] formal cycloaddition and a mild DDQ oxidation strategy. Moreover, a speculative model of the central-to-axial chirality conversion outcome was proposed based on preliminary mechanistic studies and DFT calculations. Potentially, using this strategy, useful chiral phosphine ligand can be synthesized smoothly (99% ee).


 Please wait while we load your content...
Please wait while we load your content...