Desulfonative photoredox alkylation of N-heteroaryl sulfones – an acid-free approach for substituted heteroarene synthesis†
Abstract
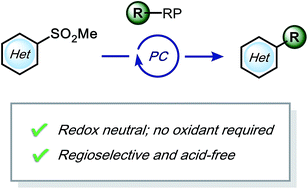
Minisci-type alkylation of electron-deficient heteroarenes has been a pivotal technique for medicinal chemists in the synthesis of drug-like molecules. However, such transformations usually require harsh conditions (e.g., strong acids, stoichiometric amount of oxidants, elevated temperatures, etc.). Herein, by utilizing photoredox catalysis, a highly-selective alkylation method using heteroaryl sulfones has been developed that can be carried out under acid-free and redox-neutral conditions. Because of these mild conditions, challenging yet privileged structures, such as monosaccharides and unprotected secondary amines, can be installed.
- This article is part of the themed collections: Most popular 2018-2019 organic chemistry articles and 2019 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...