Borohydride-containing coordination polymers: synthesis, air stability and dehydrogenation†
Abstract
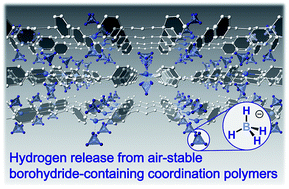
Control of the reactivity of hydride (H−) in crystal structures has been a challenge because of its strong electron-donating ability and reactivity with protic species. For metal borohydrides, the dehydrogenation activity and air stability are in a trade-off, and control of the reactivity of BH4− has been demanded. For this purpose, we synthesize a series of BH4−-based coordination polymers/metal–organic frameworks. The reactivity of BH4− in the structures is regulated by coordination geometry and neighboring ligands, and one of the compounds [Zn(BH4)2(dipyridylpropane)] exhibits both high dehydrogenation reactivity (1.4 wt% at 179 °C) and high air stability (50 RH% at 25 °C, 7 days). Single crystal X-ray diffraction analysis reveals that Hδ+⋯Hδ− dihydrogen interactions and close packing of hydrophobic ligands are the key for the reactivity and stability. The dehydrogenation mechanism is investigated by temperature-programmed desorption, in situ synchrotron PXRD and solid-state NMR.


 Please wait while we load your content...
Please wait while we load your content...