Pentanuclear iron catalysts for water oxidation: substituents provide two routes to control onset potentials†
Abstract
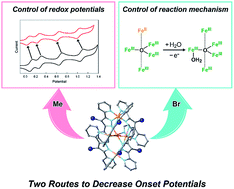
The development of robust and efficient molecular catalysts based on earth-abundant transition metals for water oxidation reactions is a challenging research target. Our group recently demonstrated the high activity and stability of a pentairon-based water oxidation electrocatalyst (M. Okamura, M. Kondo, R. Kuga, Y. Kurashige, T. Yanai, S. Hayami, V. K. K. Praneeth, M. Yoshida, K. Yoneda, S. Kawata and S. Masaoka, Nature, 2016, 530, 465–468). However, the development of strategies to decrease onset potentials for catalysis remains challenging. In this article, we report the construction of a series of pentanuclear iron complexes by introducing electron-donating (methyl) and electron-withdrawing (bromo) substituents on the ligand. Two newly synthesized complexes exhibited five reversible redox processes, similar to what is seen with the parent complex. These complexes can also serve as homogeneous catalysts for water oxidation reactions, and the faradaic efficiencies of the reactions were high. Additionally, the onset potentials of the newly developed complexes were lower than that of the parent complex. Mechanistic insights revealed that there are two methods for decreasing onset potentials: control of the redox potentials of the pentairon complex and control of the reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...