Cysteinylprolyl imide (CPI) peptide: a highly reactive and easily accessible crypto-thioester for chemical protein synthesis†
Abstract
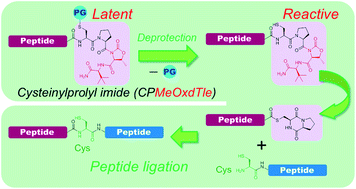
Native chemical ligation (NCL) between the C-terminal peptide thioester and the N-terminal cysteinyl-peptide revolutionized the field of chemical protein synthesis. The difficulty of direct synthesis of the peptide thioester in the Fmoc method has prompted the development of crypto-thioesters that can be efficiently converted into thioesters. Cysteinylprolyl ester (CPE), which is an N–S acyl shift-driven crypto-thioester that relies on an intramolecular O–N acyl shift to displace the amide-thioester equilibrium, enabled trans-thioesterification and subsequent NCL in one pot. However, the utility of CPE is limited because of the moderate thioesterification rates and the synthetic complexity introduced by the ester group. Herein, we develop a new crypto-thioester, cysteinylprolyl imide (CPI), which replaces the alcohol leaving group of CPE with other leaving groups such as benzimidazolidinone, oxazolidinone, and pyrrolidinone. CPI peptides were efficiently synthesized by using standard Fmoc solid-phase peptide synthesis (SPPS) and subsequent on-resin imide formation. Screening of the several imide structures indicated that methyloxazolidinone-t-leucine (MeOxd-Tle) showed faster conversion into thioester and higher stability against hydrolysis under NCL conditions. Finally, by using CPMeOxd-Tle peptides, we demonstrated the chemical synthesis of affibody via N-to-C sequential, three-segment ligation and histone H2A.Z via convergent four-segment ligation. This facile and straightforward method is expected to be broadly applicable to chemical protein synthesis.


 Please wait while we load your content...
Please wait while we load your content...