Tracking the pyrolysis process of a 3-MeOsalophen-ligand based Co2 complex for promoted oxygen evolution reaction†
Abstract
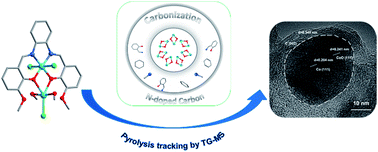
Efficient oxygen evolution reaction catalysts can be prepared via controlled pyrolysis of molecular platforms, and there is still minimal mechanistic understanding of such pyrolysis processes. Here, we introduce a 3-MeOsalophen-ligated cobalt complex as a precursor to obtain a Co-based OER electrocatalyst via controlled pyrolysis under an inert atmosphere. In our case, the unique N, O chelation mode of the 3-MeOsalophen ligand (bis[3-methoxysalicylydene]-1,2 iminophenylenediamine) was used to synthesis a Co2 complex [Co2(3-MeOsalophen)(Cl)3(CH3OH)2]. By regulating the pyrolysis conditions, we successfully obtained a N-doped carbon Co/CoOx core–shell nanostructure. More importantly, TG-MS was first adopted for tracking the decomposition products of the complex in the pyrolysis process, further finding out the evolution mechanism from Co2 to the core–shell nanostructure. As an electrocatalyst for the oxygen evolution reaction, the core–shell Co/CoOx@NC-800 nanostructure achieves an ultralow overpotential of 288 mV at 10 mA cm−2 in 1 M KOH solution. This work offers guiding insight into controlled pyrolysis via TG-MS analysis, using a novel complex precursor for precise regulation of heteroatom-doped (3d) transition metal-based electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...