Reversible perovskite electrocatalysts for oxygen reduction/oxygen evolution†
Abstract
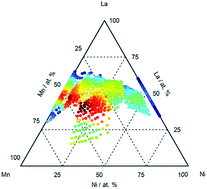
The identification of electrocatalysts mediating both the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) are prerequisite for the development of reversible fuel cells and rechargeable metal–air batteries. The question remains as to whether a bifunctional catalyst, or a single catalyst site, will exhibit potentials converging to +1.23 VRHE. Transition metal-based perovskites provide tunable catalysts where site substitution can influence both ORR and OER, however substitution in the pseudo-binary phases results in an anti-correlation in ORR and OER activities. We reveal that LaxMnyNi1−yO3−δ, compositions with lanthanum A-site sub-stoichiometry exhibit reversible activity correlating with the appearance of the Mn3+/Mn4+ redox couple. The Mn3+/Mn4+ couple is associated with Mn4+ co-existing with Mn3+ in the bulk, as La3+ is substituted by Ni2+ at the A-site to create a mixed valent system. We also show that a direct A-site substitution by the Ca2+ cation in LaxCa1−xMnyO3−δ perovskites also results in the creation of Mn4+, the appearance of the Mn3+/Mn4+ redox couple, and a concomitant reversible activity. These results highlight a general strategy of optimizing oxide electrocatalysts with reversible activity.


 Please wait while we load your content...
Please wait while we load your content...