Reductive annulations of arylidene malonates with unsaturated electrophiles using photoredox/Lewis acid cooperative catalysis†
Abstract
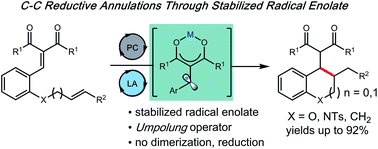
A cooperative Lewis acid/photocatalytic reduction of salicylaldehyde-derived arylidene malonates provides access to a versatile, stabilized radical anion enolate. Using these unusual umpolung operators, we have developed a novel route to access densely functionalized carbo- and heterocycles through a radical annulation addition pathway.


 Please wait while we load your content...
Please wait while we load your content...