Highly selective palladium-catalyzed one-pot, five-fold B–H/C–H cross coupling of monocarboranes with alkenes†
Abstract
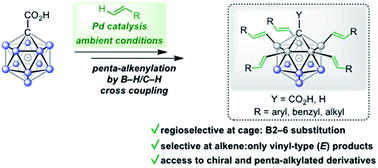
Palladium-catalyzed dehydrogenative B–H/C–H cross coupling of monocarborane anions with alkenes is reported, allowing for the first time the isolation of selectively penta-alkenylated boron clusters. The reaction cascade is regioselective for the cage positions, leading directly to B2–6 functionalization. Under mild and convenient conditions, styrenes, benzylic alkenes and aliphatic alkenes are demonstrated to be viable coupling partners with exclusive vinyl-type B–C bond formation. Multiple subsequent transformations provide access to directing group-free products, chiral derivatives and penta-alkylated cages. The five-fold coupling, combined with the latter reactions, represents a powerful methodology for the straightforward synthesis of new classes of boron clusters.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...