Enantioselective intramolecular C–H amination of aliphatic azides by dual ruthenium and phosphine catalysis†
Abstract
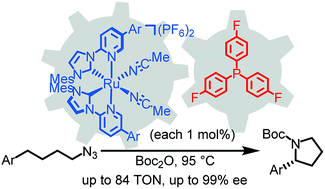
The catalytic enantioselective intramolecular C(sp3)-H amination of aliphatic azides represents an efficient method for constructing chiral saturated cyclic amines which constitute a prominent structural motif in bioactive compounds. We report a dual catalytic system involving a chiral-at-metal bis(pyridyl-NHC) ruthenium complex and tris(4-fluorophenyl)phosphine (both 1 mol%), which facilitates the cyclization of aliphatic azides to chiral α-aryl pyrrolidines with enantioselectivities of up to 99% ee, including a pyrrolidine which can be converted to the anti-tumor alkaloid (R)-(+)-crispine. Mechanistically, the phosphine activates the organic azide to form an intermediate iminophosphorane and transfers the nitrene unit to the ruthenium providing an imido ruthenium intermediate which engages in the highly stereocontrolled C–H amination. This dual catalysis combines ruthenium catalysis with the Staudinger reaction and provides a novel strategy for catalyzing enantioselective C–H aminations of unactivated aliphatic azides.
- This article is part of the themed collections: Most popular 2019-2020 catalysis articles and 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...