Hydroxy group-enabled highly regio- and stereo-selective hydrocarboxylation of alkynes†
Abstract
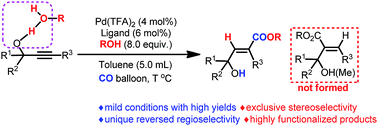
Here we present an example of utilizing hydroxy groups for regioselectivity control in the addition reaction of alkynes—a highly efficient Pd-catalyzed syn-hydrocarboxylation of readily available 2-alkynylic alcohols with CO in the presence of alcohols with an unprecedented regioselectivity affording 3-hydroxy-2(E)-alkenoates. The role of the hydroxy group has been carefully studied. The synthetic potential of the products has also been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...