Chiral cyclopentadienyl RhIII-catalyzed enantioselective cyclopropanation of electron-deficient olefins enable rapid access to UPF-648 and oxylipin natural products†
Abstract
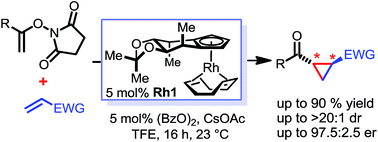
Chiral cyclopentadienyl RhIII complexes efficiently catalyze enantioselective cyclopropanations of electron-deficient olefins with N-enoxysuccinimides as the C1 unit. Excellent asymmetric inductions and high diastereoselectivities can be obtained for a wide range of substrate combinations. The reaction proceeds under mild conditions without precautions to exclude air and water. Moreover, the synthetic utility of the developed method is demonstrated by concise syntheses of members of the oxylipin natural products family and the KMO inhibitor UPF-648.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...