Efficient acceptorless photo-dehydrogenation of alcohols and N-heterocycles with binuclear platinum(ii) diphosphite complexes†
Abstract
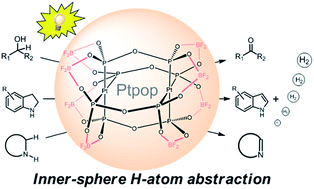
Although photoredox catalysis employing Ru(II) and Ir(III) complexes as photocatalysts has emerged as a versatile tool for oxidative C–H functionalization under mild conditions, the need for additional reagents acting as electron donor/scavenger for completing the catalytic cycle undermines the practicability of this approach. Herein we demonstrate that photo-induced oxidative C–H functionalization can be catalysed with high product yields under oxygen-free and acceptorless conditions via inner-sphere atom abstraction by binuclear platinum(II) diphosphite complexes. Both alcohols (51 examples), particularly the aliphatic ones, and saturated N-heterocycles (24 examples) can be efficiently dehydrogenated under light irradiation at room temperature. Regeneration of the photocatalyst by means of reductive elimination of dihydrogen from the in situ formed platinum(III)-hydride species represents an alternative paradigm to the current approach in photoredox catalysis.


 Please wait while we load your content...
Please wait while we load your content...