A rational quest for selectivity through precise ligand-positioning in tandem DNA-catalysed Friedel–Crafts alkylation/asymmetric protonation†
Abstract
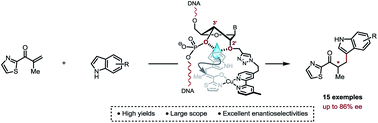
Covalent anchorage of a metallic co-factor to a DNA-based architecture is merely the only way to ensure an accurate positioning of a catalytic site within the chiral micro-environment offered by the DNA double helix. Ultimately, it also allows a fine-tuning of the catalytic pocket through simple synthetic modifications of the DNA sequence. Here, we report highly selective copper(II)-catalysed asymmetric Friedel–Crafts conjugate addition/enantioselective protonation, which is due to a careful positioning of a bipyridine ligand within a DNA framework. Most importantly, this study unveils specific structural features that account for an optimal chirality transfer from the duplex to the Friedel–Crafts adducts.


 Please wait while we load your content...
Please wait while we load your content...