Access to P-chiral phosphine oxides by enantioselective allylic alkylation of bisphenols†
Abstract
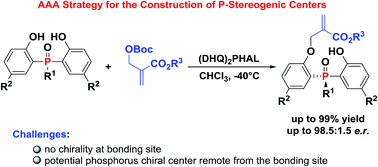
A novel biscinchona alkaloid-catalyzed highly enantioselective desymmetrization reaction of bisphenol compounds with achiral Morita–Baylis–Hillman carbonate agents was developed. Through the asymmetric allylic alkylation strategy, a broad range of optically active P-stereogenic phosphine oxides were generated with excellent to good yields (up to 99%) and high enantioselectivities (up to 98.5 : 1.5 e.r.). The reaction was further investigated by the linear free energy relationship (LFER) analysis. A possible transition state was proposed and furthered verified by theoretical calculations.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...