Donor–acceptor-stabilised germanium analogues of acid chloride, ester, and acyl pyrrole compounds: synthesis and reactivity†‡
Abstract
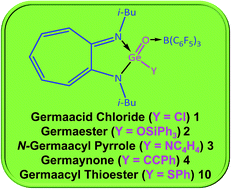
Germaacid chloride, germaester, and N-germaacyl pyrrole compounds were not known previously. Therefore, donor–acceptor-stabilised germaacid chloride (i-Bu)2ATIGe(O)(Cl) → B(C6F5)3 (1), germaester (i-Bu)2ATIGe(O)(OSiPh3) → B(C6F5)3 (2), and N-germaacyl pyrrole (i-Bu)2ATIGe(O)(NC4H4) → B(C6F5)3 (3) compounds, with Cl–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Ph3SiO–Ge
O, Ph3SiO–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C4H4N–Ge
O, and C4H4N–Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moieties, respectively, are reported here. Germaacid chloride 1 reacts with PhCCLi, KOt-Bu, and RLi (R = Ph, Me) to afford donor–acceptor-stabilised germaynone (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4), germaester (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5), and germanone (i-Bu)2ATIGe(O)(R) → B(C6F5)3 (R = Ph 6, Me 7) compounds, respectively. Interconversion between a germaester and a germaacid chloride is achieved; reaction of germaesters 2 and 5 with TMSCl gave germaacid chloride 1, and 1 reacted with Ph3SiOLi and KOt-Bu to produce germaesters 2 and 5. Reaction of N-germaacyl pyrrole 3 with thiophenol produced a donor–acceptor-stabilised germaacyl thioester (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10). Furthermore, the attempted syntheses of germaamides and germacarboxylic acids are also discussed.
O moieties, respectively, are reported here. Germaacid chloride 1 reacts with PhCCLi, KOt-Bu, and RLi (R = Ph, Me) to afford donor–acceptor-stabilised germaynone (i-Bu)2ATIGe(O)(CCPh) → B(C6F5)3 (4), germaester (i-Bu)2ATIGe(O)(Ot-Bu) → B(C6F5)3 (5), and germanone (i-Bu)2ATIGe(O)(R) → B(C6F5)3 (R = Ph 6, Me 7) compounds, respectively. Interconversion between a germaester and a germaacid chloride is achieved; reaction of germaesters 2 and 5 with TMSCl gave germaacid chloride 1, and 1 reacted with Ph3SiOLi and KOt-Bu to produce germaesters 2 and 5. Reaction of N-germaacyl pyrrole 3 with thiophenol produced a donor–acceptor-stabilised germaacyl thioester (i-Bu)2ATIGe(O)(SPh) → B(C6F5)3 (10). Furthermore, the attempted syntheses of germaamides and germacarboxylic acids are also discussed.


 Please wait while we load your content...
Please wait while we load your content...