Tandem Peterson olefination and chemoselective asymmetric hydrogenation of β-hydroxy silanes†
Abstract
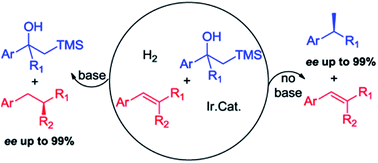
Here, we report the first Ir–N,P complex catalyzed tandem Peterson olefination and asymmetric hydrogenation of β-hydroxy silanes. This reaction resulted in the formation of chiral alkanes in high isolated yields (up to 99%) and excellent enantioselectivity (up to 99% ee) under mild conditions. Modification of the reaction conditions provides a choice to transform either an olefin or the β-hydroxy silane in a chemoselective manner. Additionally, based on this method, an expedient enantioselective synthesis of (S)-(+)-α-curcumene, from a simple ketone, was accomplished in two steps with 75% overall yield and 95% ee.


 Please wait while we load your content...
Please wait while we load your content...