Cooperativity basis for small-molecule stabilization of protein–protein interactions†
Abstract
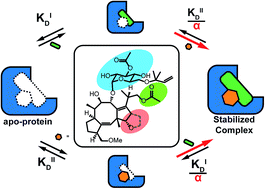
A cooperativity framework to describe and interpret small-molecule stabilization of protein–protein interactions (PPI) is presented. The stabilization of PPIs is a versatile and emerging therapeutic strategy to target specific combinations of protein partners within the protein interactome. Currently, the potency of PPI stabilizers is typically expressed by their apparent affinity or EC50. Here, we propose that the effect of a PPI stabilizer be best described involving the cooperativity factor, α, between the stabilizer and binding partners in addition to the intrinsic affinity, KDII, of the stabilizer for one of the apo-proteins. By way of illustration, we combine fluorescence polarization measurements with thermodynamic modeling to determine the α and KDII for the PPI stabilization of 14-3-3 and TASK3 by fusicoccin-A (FC-A) and validate our approach by studying other PPI-partners of 14-3-3 proteins. Finally, we characterize a library of different stabilizer compounds, and perform structure–activity relationship studies in which molecular changes could be attributed to either changes in cooperativity or intrinsic affinity. Such insights should aid in the development of more effective protein–protein stabilizer drugs.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...