Perfluoroalkylative pyridylation of alkenes via 4-cyanopyridine-boryl radicals†
Abstract
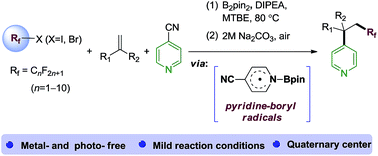
A metal-free and photo-free method for the perfluoroalkylative pyridylation of alkenes has been developed via a combination of computational and experimental studies. Density functional theory calculations and control experiments indicate that the homolysis of Rf−X (X = Br, I) bonds by the 4-cyanopyridine-boryl radicals in situ generated from 4-cyanopyridine and B2pin2 is the key step. Sequential addition of Rf radicals to alkenes and the selective cross-coupling of the resulting alkyl radicals and 4-cyanopyridine-boryl radicals gives alkene difunctionalization products with a quaternary carbon center. This method exhibits a broad substrate scope and good functional group compatibility.
- This article is part of the themed collection: Celebrating a century of chemical excellence at Nanjing University


 Please wait while we load your content...
Please wait while we load your content...