Dithiane-directed Rh(iii)-catalyzed amidation of unactivated C(sp3)–H bonds†
Abstract
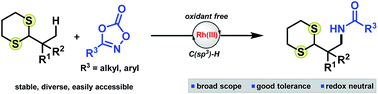
An oxidant-free Rh(III)-catalyzed direct amidation of alkyl dithianes via C(sp3)–H bond activation utilizing diverse and robust dioxazolone reagents is reported. The reaction hinges on use of a Cp*Rh(III) complex in combination with an essential amino-carboxylate additive to generate usefully protected 1,3-aminoaldehyde derivatives. The scalability of the reaction was demonstrated as was a series of downstream product functionalizations, including dithiane deprotection, anion alkylation and reductive desulfurization, highlighting the general applicability of this transformation in the synthesis of novel scaffolds and building blocks.
- This article is part of the themed collections: Most popular 2019-2020 catalysis articles and 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...