Monomeric Cp3tAl(i): synthesis, reactivity, and the concept of valence isomerism†
Abstract
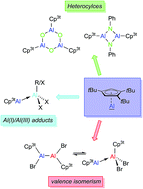
With the isolation of Cp3tAl (1), the first monomeric Cp-based Al(I) species could be realized in a pure form via a three-step reaction sequence (salt elimination/adduct formation/adduct cleavage) starting from readily available AlBr3. Due to its monomeric structure, reactions involving 1 were found to proceed more selectively, faster, and under milder conditions than for tetrameric (Cp*Al)4. Thus, 1 readily formed simple Lewis acid–base adducts with tBuAlCl2 (6) and AlBr3 (7), reactions that before have always been interfered with by the presence of aluminum halide bonds. In addition, the 2 : 1 reaction of 1 with AlBr3 enabled the realization of the very rare trialuminum adduct species 8. 1 also reacted rapidly with N2O and PhN3 at room temperature to afford Al3O3 and Al2N2 heterocycles 9 and 10, respectively. With the structural characterization of products 4 and 5, the reaction of monovalent 1 with Cp3tAlBr2 (2) provided the first experimental evidence for the concept of valence isomerism between dialanes and their Al(I)/Al(III) Lewis adducts.


 Please wait while we load your content...
Please wait while we load your content...