Frontier molecular orbital effects control the hole-catalyzed racemization of atropisomeric biaryls†
Abstract
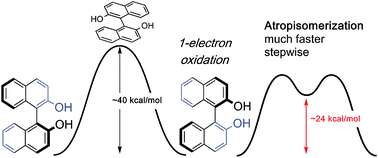
Atropisomeric biaryl systems are privileged architectures used in asymmetric synthesis and pharmaceutical structures. We report that by simply removing a single-electron, the resistance of biaryls towards racemization is reduced dramatically. Even though the steric properties are unaltered, biaryl oxidation changes atropisomerization into a two step mechanism with considerably smaller activation barriers than closed-shell biaryls. The effect is general for a series of biaryls and helicenes studied and results from the dependence of frontier molecular orbital energies on biaryl conformation.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...