Real-time mechanistic study of carbon nanotube anion functionalisation through open circuit voltammetry†
Abstract
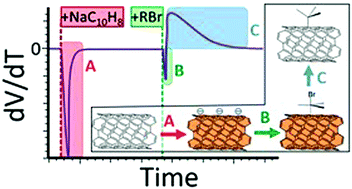
The mechanism of the functionalisation of reduced single walled carbon nanotubes with organobromides was monitored by open circuit voltammetry throughout the reaction and further elucidated through a series of comparative reactions. The degree of functionalisation was mapped against the reagent reduction potential, degree of electron donation of substituents (Hammett parameter), and energies calculated, ab initio, for dissociation and heterolytic cleavage of the C–Br bond. In contrast to the previously assumed reduction/homolytic cleavage mechanism, the reaction was shown to consist of a rapid association of carbon–halide bond to the reduced nanotube as a complex, displacing surface-condensed countercations, leading to an initial increase in the net nanotube surface negative charge. The complex subsequently slowly degrades through charge transfer from the reduced single-walled carbon nanotube to the organobromide, utilizing charge, and the carbon–halide bond breaks heterolytically. Electron density on the C–Br bond in the initial reagent is the best predictor for degree of functionalisation, with more electron donating substituents increasing the degree of functionalisation. Both the mechanism and the new application of OCV to study such reactions are potentially relevant to a wide range of related systems.


 Please wait while we load your content...
Please wait while we load your content...