Reversible alkene binding and allylic C–H activation with an aluminium(i) complex†
Abstract
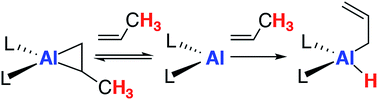
The monomeric molecular aluminium(I) complex 1 [{(ArNCMe)2CH}Al] (Ar = 2,6-di-iso-propylphenyl) reacts with a series of terminal and strained alkenes including ethylene, propylene, allylbenzene and norbornene to form alkene bound products. Remarkably all these reactions are reversible under mild conditions (298–353 K) with alkene binding being disfavoured at higher temperatures due to the positive reaction entropy. Van't Hoff analyses have allowed quantification of the binding events with 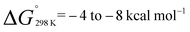 . Calculations and single crystal X-ray diffraction studies are consistent with the alkene bound species being metallocyclopropane complexes. Alkene binding involves a reversible redox process with changes from the +1 to +3 aluminium oxidation state. Under more forcing conditions the metallocyclopropane complexes undergo non-reversible allylic C–H bond activation to generate aluminium(III) allyl hydride complexes. This represents a rare example of redox-based main group reactivity in which reversible substrate binding is followed by a further productive bond breaking event. Analysis of the mechanism reveals a reaction network in which alkene dissociation and reformation of 1 is required for allylic C–H activation, a realisation that has important implications for the long-term goal of developing redox-based catalytic cycles with main group compounds.
. Calculations and single crystal X-ray diffraction studies are consistent with the alkene bound species being metallocyclopropane complexes. Alkene binding involves a reversible redox process with changes from the +1 to +3 aluminium oxidation state. Under more forcing conditions the metallocyclopropane complexes undergo non-reversible allylic C–H bond activation to generate aluminium(III) allyl hydride complexes. This represents a rare example of redox-based main group reactivity in which reversible substrate binding is followed by a further productive bond breaking event. Analysis of the mechanism reveals a reaction network in which alkene dissociation and reformation of 1 is required for allylic C–H activation, a realisation that has important implications for the long-term goal of developing redox-based catalytic cycles with main group compounds.
- This article is part of the themed collections: Most popular 2019-2020 inorganic, main group and crystal engineering chemistry articles and Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...