A catalytic antioxidant for limiting amyloid-beta peptide aggregation and reactive oxygen species generation†
Abstract
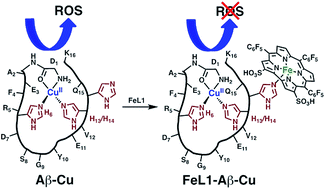
Alzheimer's disease (AD) is a multifaceted disease that is characterized by increased oxidative stress, metal-ion dysregulation, and the formation of intracellular neurofibrillary tangles and extracellular amyloid-β (Aβ) aggregates. In this work we report the large affinity binding of the iron(III) 2,17-bis-sulfonato-5,10,15-tris(pentafluorophenyl)corrole complex FeL1 to the Aβ peptide (Kd ∼ 10−7) and the ability of the bound FeL1 to act as a catalytic antioxidant in both the presence and absence of Cu(II) ions. Specific findings are that: (a) an Aβ histidine residue binds axially to FeL1; (b) that the resulting adduct is an efficient catalase; (c) this interaction restricts the formation of high molecular weight peptide aggregates. UV-Vis and electron paramagnetic resonance (EPR) studies show that although the binding of FeL1 does not influence the Aβ–Cu(II) interaction (Kd ∼ 10−10), bound FeL1 still acts as an antioxidant thereby significantly limiting reactive oxygen species (ROS) generation from Aβ-Cu. Overall, FeL1 is shown to bind to the Aβ peptide, and modulate peptide aggregation. In addition, FeL1 forms a ternary species with Aβ–Cu(II) and impedes ROS generation, thus showing the promise of discrete metal complexes to limit the toxicity pathways of the Aβ peptide.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...