Low overpotential water oxidation at neutral pH catalyzed by a copper(ii) porphyrin†
Abstract
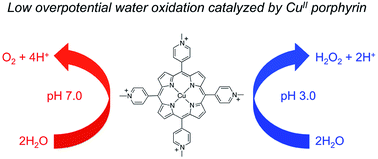
Low overpotential water oxidation under mild conditions is required for new energy conversion technologies with potential application prospects. Extensive studies on molecular catalysis have been performed to gain fundamental knowledge for the rational designing of cheap, efficient and robust catalysts. We herein report a water-soluble CuII complex of tetrakis(4-N-methylpyridyl)porphyrin (1), which catalyzes the oxygen evolution reaction (OER) in neutral aqueous solutions with small overpotentials: the onset potential of the catalytic water oxidation wave measured at current density j = 0.10 mA cm−2 is 1.13 V versus a normal hydrogen electrode (NHE), which corresponds to an onset overpotential of 310 mV. Constant potential electrolysis of 1 at neutral pH and at 1.30 V versus NHE displayed a substantial and stable current for O2 evolution with a faradaic efficiency of >93%. More importantly, in addition to the 4e water oxidation to O2 at neutral pH, 1 can catalyze the 2e water oxidation to H2O2 in acidic solutions. The produced H2O2 is detected by rotating ring–disk electrode measurements and by the sodium iodide method after bulk electrolysis at pH 3.0. This work presents an efficient and robust Cu-based catalyst for water oxidation in both neutral and acidic solutions. The observation of H2O2 during water oxidation catalysis is rare and will provide new insights into the water oxidation mechanism.
- This article is part of the themed collections: Most popular 2019-2020 catalysis articles, Most popular 2018-2019 catalysis articles, In celebration of Chinese New Year and 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...