Synthesis and structural features of thiophene-fused analogues of warped nanographene and quintuple helicene†
Abstract
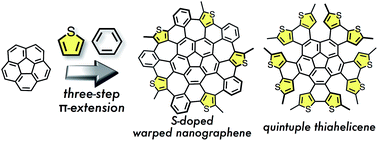
Thiophene-fused analogues of warped nanographene (WNG) and quintuple helicene (QH) were synthesized via a three-step π-extension of corannulene. Similar to the synthetic route to WNG, five hexagons and five heptagons were generated by a Scholl reaction of pentakis(thienylphenyl)corannulene to form pentathiaWNG. In contrast, decathiaWNG could not be obtained from pentakis(thienylthienyl)corannulene, and instead decathiaQH was generated from the photocyclization of the precursor. X-ray crystallography of the products revealed their conformations and packing modes in the solid state. The configurational features of decathiaQH were further examined by DFT calculations. The absorption and fluorescence spectra of the sulfur-containing WNG and QH were shifted relative to those of the corresponding sulfur-free analogues.
- This article is part of the themed collection: Most popular 2018-2019 nanoscience articles


 Please wait while we load your content...
Please wait while we load your content...