Reductive α-borylation of α,β-unsaturated esters using NHC–BH3 activated by I2 as a metal-free route to α-boryl esters†
Abstract
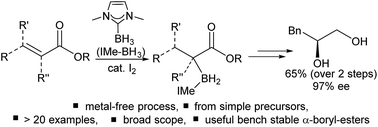
Useful α-boryl esters can be synthesized in one step from α,β-unsaturated esters using just a simple to access NHC–BH3 (NHC = N-heterocyclic carbene) and catalytic I2. The scope of this reductive α-borylation methodology is excellent and includes a range of alkyl, aryl substituted and cyclic and acyclic α,β-unsaturated esters. Mechanistic studies involving reductive borylation of a cyclic α,β-unsaturated ester with NHC–BD3/I2 indicated that concerted hydroboration of the alkene moiety in the α,β-unsaturated ester proceeds instead of a stepwise process involving initial 1,4-hydroboration; this is in contrast to the recently reported reductive α-silylation. The BH2(NHC) unit can be transformed into electrophilic BX2(NHC) moieties (X = halide) and the ester moiety can be reduced to the alcohol with the borane unit remaining intact to form β-boryl alcohols. The use of a chiral auxiliary, 8-phenylmenthyl ester, also enables effective stereo-control of the newly formed C–B bond. Combined two step ester reduction/borane oxidation forms diols, including excellent e.e. (97%) for the formation of S-3-phenylpropane-1,2-diol. This work represents a simple transition metal free route to form bench stable α-boryl esters from inexpensive starting materials.


 Please wait while we load your content...
Please wait while we load your content...