Anion control of tautomeric equilibria: Fe–H vs. N–H influenced by NH⋯F hydrogen bonding†
Abstract
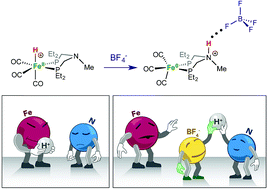
Counterions can play an active role in chemical reactivity, modulating reaction pathways, energetics and selectivity. We investigated the tautomeric equilibrium resulting from protonation of Fe(PEtNMePEt)(CO)3 (PEtNMePEt = (Et2PCH2)2NMe) at Fe or N. Protonation of Fe(PEtNMePEt)(CO)3 by [(Et2O)2H]+[B(C6F5)4]− occurs at the metal to give the iron hydride [Fe(PEtNMePEt)(CO)3H]+[B(C6F5)4]−. In contrast, treatment with HBF4·OEt2 gives protonation at the iron and at the pendant amine. Both the FeH and NH tautomers were characterized by single crystal X-ray diffraction. Addition of excess BF4− to the equilibrium mixture leads to the NH tautomer being exclusively observed, due to NH⋯F hydrogen bonding. A quantum chemical analysis of the bonding properties of these systems provided a quantification of hydrogen bonding of the NH to BF4− and to OTf−. Treatment of Fe(PEtNMePEt)(CO)3 with excess HOTf gives a dicationic complex where both the iron and nitrogen are protonated. Isomerization of the dicationic complex was studied by NOESY NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...