Emergence of high ORR activity through controlling local density-of-states by alloying immiscible Au and Ir†
Abstract
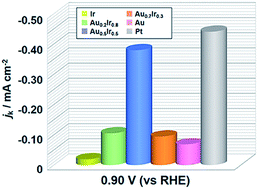
The electronic structure of surface atoms has a great effect on catalytic activity because the binding energy of reactants is closely related to the electronic structure. Therefore, designing and controlling the local density of states (LDOS) of the catalyst surface would be a rational way to develop innovative catalysts. Herein, we first demonstrate a highly active AuIr solid-solution alloy electrocatalyst for the oxygen reduction reaction (ORR) by emulating the Pt LDOS profile. The calculated LDOS of Ir atoms on the Au0.5Ir0.5(111) surface closely resembled that of Pt(111), resulting in suitable oxygen adsorption energy on the alloy surface for the ORR. We successfully synthesized AuIr solid-solution alloys, while Ir and Au are immiscible even above their melting points in the bulk state. Although monometallic Ir or Au is not active for the ORR, the synthesized Au0.5Ir0.5 alloy demonstrated comparable activity to Pt at 0.9 V versus a reversible hydrogen electrode.
- This article is part of the themed collection: Editor’s Choice – Toshiharu Teranishi


 Please wait while we load your content...
Please wait while we load your content...