A divergent synthetic pathway for pyrimidine-embedded medium-sized azacycles through an N-quaternizing strategy†
Abstract
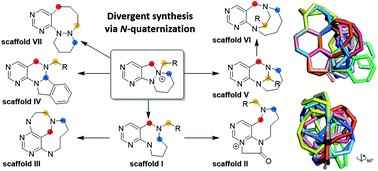
Medium-sized heterocycles have recently received significant attention because of their potential roles as modulators of protein–protein interactions, but their molecular diversity and synthetic availability are still inadequate to meet the demand. To address these issues, we developed a new divergent synthetic pathway for skeletally distinct pyrimidine-containing medium-sized azacycles. We introduced N-quaternized pyrimidine-containing polyheterocycles as novel key intermediates for diversity-generating reactions via selective bond cleavages or migrations and prepared 14 discrete core skeletons in an efficient manner. The skeletal diversity of the resulting molecular frameworks was confirmed by chemoinformatic analysis.
- This article is part of the themed collection: Celebrating Chemical Science in Korea


 Please wait while we load your content...
Please wait while we load your content...