Tuning the electronic properties of hexanuclear cobalt sulfide superatoms via ligand substitution†
Abstract
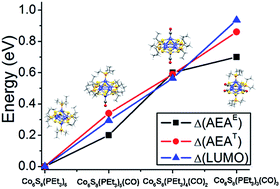
Molecular clusters are attractive superatomic building blocks for creating materials with tailored properties due to their unique combination of atomic precision, tunability and functionality. The ligands passivating these superatomic clusters offer an exciting opportunity to control their electronic properties while preserving their closed shells and electron counts, which is not achievable in conventional atoms. Here we demonstrate this concept by measuring the anion photoelectron spectra of a series of hexanuclear cobalt sulfide superatomic clusters with different ratios of electron-donating and electron-withdrawing ligands, Co6S8(PEt3)6−x(CO)x (x = 0–3). We find that Co6S8(PEt3)6 has a low electron affinity (EA) of 1.1 eV, and that the successive replacement of PEt3 ligands with CO gradually shifts its electronic spectrum to lower energy and increases its EA to 1.8 eV. Density functional theory calculations reveal that the increase of EA results from a monotonic lowering of the cluster highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO). Our work provides unique insights into the electronic structure and tunability of superatomic building blocks.


 Please wait while we load your content...
Please wait while we load your content...